Seamless Integration of DI²C Communication in Medical Product Development
December 2, 2024
Table of contents
DI²C communication provides a practical solution in embedded systems, delivering reliable, high-performance connectivity for industries like medical product development. In the medical field, where electronics play a critical role in diagnosis, treatment, and monitoring, dI²C enables efficient communication across systems.
This article explores how dI²C communication enhances medical product development by outlining its benefits, applications, and impact on project timelines.
What is DI²C and How Does it Work?
DI²C stands for Differential I²C, an advanced version of the standard Inter-Integrated Circuit (I²C) protocol. Unlike the original I²C, which operates at a single-ended configuration within a single PCB, dI²C expands its functionality by providing differential communication.
This allows development teams to connect multiple PCBs, enabling data transmission across extended distances without altering the existing microprocessor code.
Key Features of DI²C:
- Differential signaling: By using differential signaling, DI²C minimizes noise interference, providing a robust communication link, even in environments prone to electrical interference.
- Extended reach: dI²C supports long-distance communication, making it ideal for complex medical devices with multiple boards and sensors.
- Backward compatibility: dI²C can work with existing I²C systems, offering seamless integration with minimal hardware changes.
- No code modifications: Unlike other communication technologies, dI²C does not require rewriting or adjusting firmware, allowing for quicker deployment.
Benefits of DI²C in Medical Product Development
1. Seamless Multiboard Communication Without Firmware Changes
One of the major advantages of dI²C for medical product manufacturers is its ability to facilitate communication across multiple boards without a need to change the existing firmware.
Many medical devices are composed of several interconnected PCBs, each serving a different function (e.g., monitoring, control, or user interface). With dI²C, developers can integrate additional boards or extend connections without additional coding, saving significant time and resources during product development.
2. Enhanced Reliability Through Differential Signaling
DI²C’s differential signaling technology provides a robust and stable connection in medical devices where signal integrity and reliability are paramount. Differential signaling helps to counteract electrical noise, which is essential in medical environments with high interference potential, such as hospitals or clinical settings.
This feature ensures that data is accurately transmitted between different components, minimizing the risk of data loss or signal corruption, which is critical in life-saving medical applications.
3. Flexibility in System Design
By enabling cross-PCB communication, dI²C allows for greater flexibility in system design. Development teams are no longer confined to single-board setups, instead, they can develop modular, multi-board configurations that allow individual components to be swapped, updated, or scaled as needed.
This modularity is particularly beneficial for medical product iterations and upgrades, where adapting to regulatory standards or accommodating new features is essential.
4. Reduced Development Time and Cost
With dI²C, companies can reduce time-to-market by eliminating the need for custom code modifications when expanding system connections. Reduced engineering and debugging efforts translate to lower development costs, a critical consideration for medical device manufacturers operating under tight budgets and timelines.
Furthermore, this streamlined process accelerates prototyping and testing phases, allowing teams to focus on enhancing product performance and reliability.
5. Compatibility with Existing I²C Protocols
For medical device developers who have invested in I²C infrastructure, dI²C offers a seamless upgrade path, without overhauling their existing infrastructure. dI²C’s compatibility with traditional I²C means that it works within the current hardware setup while minimizing retraining, infrastructure changes, and disruptions, making it a cost-effective solution.
Applications of DI²C Communication in Medical Devices
Patient Monitoring Systems
Modern patient monitoring systems involve numerous sensors distributed across different parts of the body, often connecting to various boards and systems for comprehensive health data monitoring. With dI²C, these sensors can communicate efficiently without interference or data loss, ensuring reliable, real-time monitoring essential for patient safety.
Diagnostic Equipment
For diagnostic equipment such as MRI and ultrasound machines, signal clarity is crucial. dI²C’s noise-resistant differential communication guarantees accurate data transfer between boards, improving the performance of complex imaging and diagnostic equipment by reducing potential errors.
Wearable Health Technology
The wearable health technology market has seen significant growth, with dI²C helping manufacturers design multi-board systems that remain lightweight and power-efficient.
In fitness trackers or medical wearables, dI²C enables smooth inter-board communication, supporting advanced monitoring features like heart rate, oxygen levels, and ECG without compromising device design.
How DI²C Ensures Compliance in Medical Product Development
Compliance is a critical aspect of medical device development, involving strict adherence to health, safety, and performance standards. DI²C’s inherent reliability and precision help meet stringent regulatory requirements for medical device performance.
Signal Integrity and Data Accuracy
Medical devices must maintain high standards of data accuracy, as even slight errors can lead to misdiagnoses or incorrect treatments. The differential signaling in dI²C reduces interference and ensures consistent data integrity, which is crucial for meeting regulatory requirements on signal reliability and performance.
Simplified Validation and Testing
DI²C allows manufacturers to conduct validation and testing with fewer variables. Since the protocol is compatible with existing I²C infrastructures and requires no code changes, development teams can focus on testing the device’s performance without concerns about firmware conflicts or additional testing cycles for communication.
This simplification reduces complexity during regulatory audits and ISO certification, easing compliance verification.
Accelerating Time-to-Market with DI²C Technology
Time-to-market is a critical factor in medical product development. DI²C’s unique capabilities enable companies to significantly shorten development cycles by allowing multi-board communication without extra firmware development. This not only accelerates initial product launches but also enables faster iterations for existing products.
Prototype to Production in Record Time
DI²C’s ease of implementation allows manufacturers to move from prototype to production quickly, enhancing responsiveness to market demands. Medical devices, often required urgently, benefit from this speed as it ensures devices can reach hospitals and clinics faster, fulfilling critical healthcare needs.
By simplifying multi-board communication, dI²C accelerates the transition from prototype to production. This agility enables manufacturers to quickly respond to market demands and deliver critical healthcare solutions promptly.
Streamlining Production for Consistent Quality
In addition to expediting development, dI²C simplifies the manufacturing process itself. By eliminating the need for additional components for new PCB connections, dI²C supports lean manufacturing practices. This results in improved production efficiency while maintaining the consistent quality essential for medical devices.
Case Study: Enhancing DNA Diagnostics with DI²C
In a recently concluded project by the engineers at EKTOS, the Development team worked on a device that employs Loop-Mediated Isothermal Amplification (LAMP). It is a highly efficient, single-tube technique for amplifying DNA to facilitate rapid and accurate diagnostics. (see Figure 1).
The diagnostic process utilizes multiplex fluorescence-based LAMP assays housed in specialized sample strips prepared by trained lab technicians. These assays incorporate fluorescent dyes that selectively bind to double-stranded DNA and emit fluorescence when exposed to light. The device continuously monitors the fluorescence signal throughout the reaction, enabling real-time detection of DNA amplification. This dynamic feedback allows the quantification of target DNA based on fluorescence intensity, which correlates directly with the reaction’s progress and the DNA concentration in the sample.
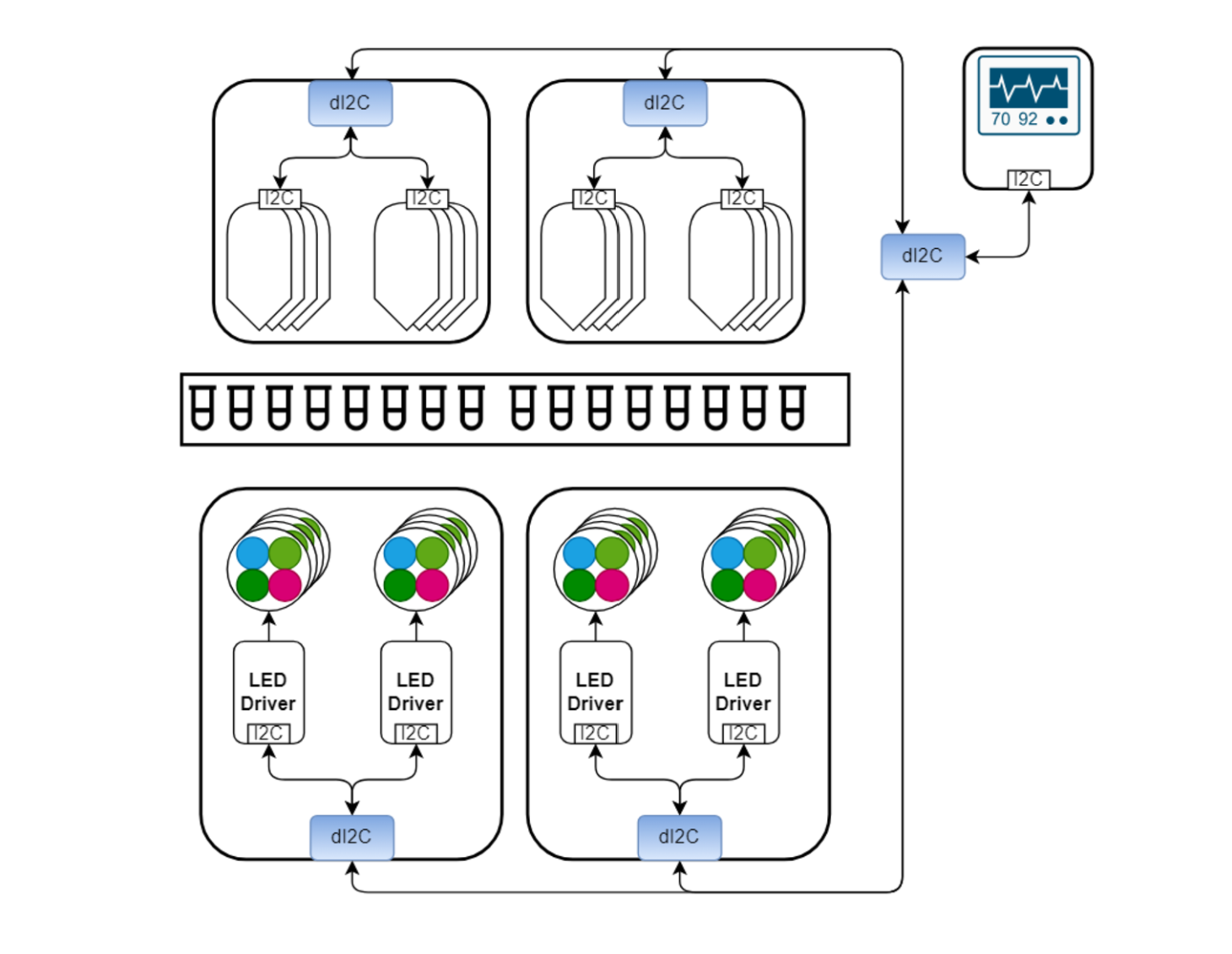 Figure 1: Device Concept
Figure 1: Device Concept
From a technical perspective, the system architecture involves over 20 integrated circuits (ICs), each equipped with an I²C interface. These ICs include sensors and PWM controllers that regulate the LEDs used to excite the fluorescent dyes. The use of I²C enables centralized control and data collection, streamlining device operation while minimizing hardware complexity. By leveraging distributed I²C nodes, the design eliminates the need for additional microcontrollers, optimizing data acquisition and transmission via protocols like UART for seamless integration.
The I²C interface could not be used as a communication between the nodes for the medical device due to the noise and distance from board to board, but in this case, the differential I²C solves these issues and perfectly fits into that.
This sophisticated design addresses critical challenges in molecular diagnostics and showcases how dI²C can enhance complex medical systems with robust, scalable, and efficient communication.
Transform Your Medical Devices with DI²C
EKTOS specializes in reliable and compliant communication technologies for medical devices, with expertise in dI²C integration. Whether you’re developing new solutions or upgrading existing systems, dI²C can help streamline processes, reduce costs, and enhance performance.
Contact the EKTOS Development team to learn how dI²C can enhance your medical devices.